Blockchain for Healthcare and Research Data Governance
Blockchain can be used to create cryptographically validated data governance strategies while maintaining data privacy.
The governance of data used for biomedical research and clinical trials is an important requirement for generating accurate results. To improve the visibility of data quality and analysis, we developed TrialChain, a blockchain-based platform that can be used to validate data integrity from large, biomedical research studies. More information about TrialChain can be found in our arXiv article and the code is publicly available on the TrialChain GitHub repository.

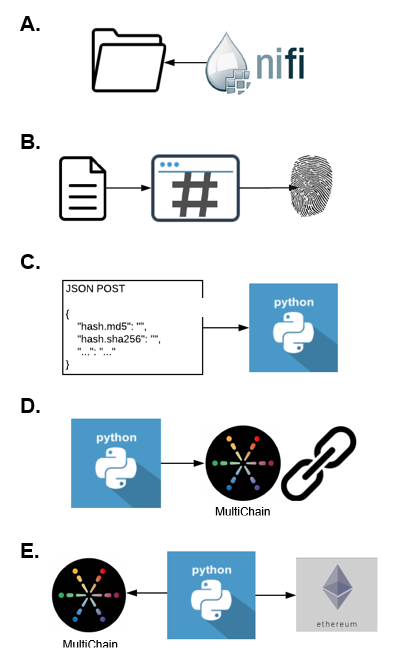
TrialChain Workflow
- A NiFi workflow monitors source systems for new files/data assets (A) which are hashed (B) to create a cryptographic digital fingerprint of the file
- The hash, file metadata, and timestamp are then sent to a web service (C) which logs the data into a private blockchain (D)
- Periodically, a Python script obtains the current block hash from the private blockchain and submits it to the public Ethereum network (E) so file states and timestamps can be publicly audited